Batteries are a practical means of supplying electricity to both small and large dc devices in the plant. They are used in equipment ranging from handheld calculators and flashlights to forklift trucks and mine cars.
This article explains what a cell is, the difference between the various types of cells, constructions and characteristics of cells, and how to store and maintain them. As well as how batteries are rated, and how to select the correct battery or combination of cells for a particular application.
Battery Cells
A cell is a container with two electrodes and an electrolyte. The cell can be used either for producing electricity by chemical action or for using electricity in electrolysis.
In the voltaic cell, a chemical reaction between the electrodes and the electrolyte creates an excess of electrons on the cathode and a deficiency of electrons on the anode. This imbalance creates a potential difference between the two electrodes.
Two basic kinds of electric cells are used in commercial and industrial applications.
• The primary cell must be thrown away after it is used up. It cannot be recharged or used again.
• The Secondary cell can be recharged and used again.
Both kinds of cells can be either wet or dry. That is, the electrolyte can be either a liquid or a moist paste.
A primary cell is an electric cell that cannot be recharged after the chemicals inside have been used up in producing electricity. For many years the primary cell was the only source of electric current.
The secondary cell can be recharged. For this reason, it is also called a storage cell. In the secondary cell, the electrodes change to somewhat different materials as the cell discharges. The electrodes can be restored to their original materials by forcing an electric current through the cell in the opposite direction.
Originally, wet cells were the only practical source of electricity. They are still widely used today. However, they have the disadvantage of being difficult to carry, because they are usually quite heavy and the liquid electrolyte can spill. For these reasons, wet cells have been replaced by dry cells for many uses.
One kind of wet cell is the Leclanche cell. It had an important influence on the development of battery technology. In the electrochemical action of the Leclanche cell, the carbon anode gives up electrons to form chloride ions (Cl-) in the electrolyte. The chloride ions drift toward the zinc cathode. As they arrive, zinc atoms leave the cathode to form zinc ions, making a solution of zinc chloride. The zinc cathode is destroyed by this chemical action.
Manganese dioxide mixed into the electrolyte of the Leclanche cell prevents polarization. Polarization is the formation of hydrogen bubbles on the carbon anode. They restrict the operation of the cell. Today, improved forms of the wet cell have replaced the Leclanche cell. However, the basic principles of operation remain the same.
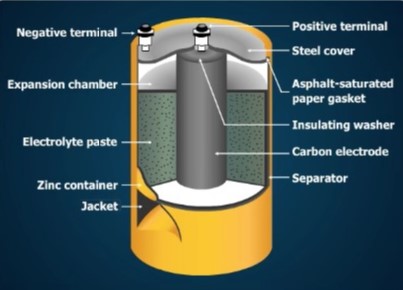
Today the dry cell is the most widely used primary cell. The only basic difference between a Leclanche wet cell and a dry cell is that the electrolyte in a dry cell is a paste and in a wet cell it is a liquid.
Dry cells are typically used for supplying low-voltage direct current. Examples of their uses range from small pen lights to providing power for test instruments and emergency lighting equipment.
Dry cells have several advantages over wet cells.
• They are portable, without the problem of spilling.
• They are inexpensive.
• They are reliable when used under the proper conditions.
Dry cells also have certain disadvantages. Two of these disadvantages are especially important.
• They do not have a long storage life.
• They work best in household devices rather than for supplying a steady load.
Construction of Dry Cells
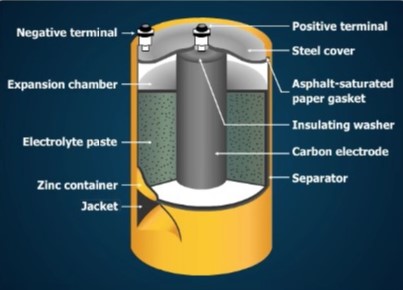
A No. 6 dry cell container is made of zinc and serves as the negative electrode. The container is sealed with a waterproof cover to protect the cell from leakage of the electrolyte and to retain its moisture. If the electrolyte dries out, electrochemical action stops. Dry cells are often enclosed in a strong steel jacket to prevent leakage caused by pressure inside a discharged cell.
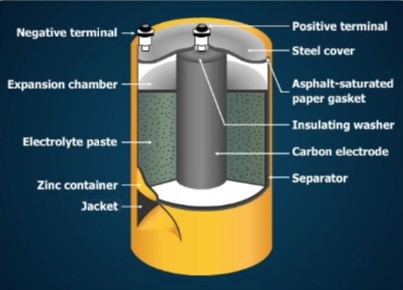
Space is provided at the top of the cell to permit expansion of the paste. The paste expands as gas forms during operation. The parts of the cell are insulated from one another by gaskets and separators to prevent internal short circuits.
Terminal posts at the top permit easy connection of the cell to other cells or to a load. Other kinds of cells have different kinds of terminals. Some cells have a button-shaped positive terminal on the top, and a flat negative terminal at the bottom. Others have snaps on the top that connect to matching snaps on the load. The terminals may be marked + or –.
Characteristics of Dry Cells
The potential difference between the terminals of a dry cell is about 1.5 volts (abbreviated V), regardless of the size of the cell. The potential difference depends only on the chemicals used in making the cell. The size affects only the length of time required for discharge. The larger the volume of a dry cell, the longer its life for a given load. The life is longer because the cell has a greater capacity for chemicals. Increasing the volume of chemicals provides more time before the chemicals are used up.
Higher potential differences can be achieved by connecting two or more dry cells in series. A typical flashlight, for example, may need a potential difference of 3V across the filament of the bulb. This potential difference is obtained by connecting two l.5V dry cells in series. To obtain 6V, you need four 1.5V cells. A 9V “transistor radio battery” has 6 cells inside, each of which produces 1.5V.
The life of a dry cell depends to a great extent on the rate of discharge. Most dry-cell applications require only small amounts of current for short periods of time. If the cell is required to furnish current for a long time, its life will be shortened considerably—even if the current is quite low.
Although both the AAA and No. 6 dry cells are 1.5 V, there is a considerable difference in the amount of current and the length of service each can provide. The difference in life is due to the difference in volume. Smaller cells deliver less current because they have a higher internal resistance.
You may have noticed that the light from a flashlight is quite bright when you first switch it on. But the light becomes dimmer after a while. If you switch the flashlight off, let the cells “rest” for half an hour or so, and then turn it on again, the light again seems to be as bright as when you first turned it on.
The reason for this behavior is that the cell becomes polarized by hydrogen and ammonia gases during operation. The gases interfere with the movement of ions in the electrolyte and slow down the electrochemical action.
The polarization is reduced by manganese dioxide in the electrolyte. But the gases collect faster than the depolarizer can remove or absorb them. However, when you turn the flashlight off, the depolarizer continues to remove the gases from the carbon electrode until polarization is no longer a problem when you turn it back on.
The temperature of a dry cell affects its performance. Most dry cells are designed to operate best at a temperature of about 21°C (about 70°F).
• A higher temperature increases the cell’s current capacity, but it
shortens the life of the cell because the electrolyte dries out faster.
• A lower temperature reduces the cell’s current capacity, because the ions in the electrolyte cannot move as rapidly from one plate to the other.
Care and Maintenance of Dry Cells
Dry cells require only a minimum of maintenance. All that is required is the removal of discharged cells and replacement with new cells. Removing discharged cells promptly prevents possible damage to the instrument or other device due to corrosion caused by a leaking cell.
The most important precaution to take with dry cells is to store them properly. A dry cell can get weak during storage, even though it is never used, because of local action (internal short-circuiting). Dry cells should be stored in a dry place at a temperature of about 20°C (about 70°F) to preserve their useful life.
As a general rule, the smaller the dry cell, the shorter its storage life. Good-quality No. 6 dry cells normally have a storage life of at least a year. Smaller dry cells may be useless after only a few months of storage. Some dry cells are dated to indicate how soon they should be used.
A new No. 6 dry cell should supply at least 25 amperes (abbreviated A) through a short circuit. A cell that has been in service should be able to supply at least 10 A if it is to remain in service. Measurements of the current through a short circuit should be conducted for very short intervals only.
You can test a dry cell with an ammeter and a voltmeter.
• The size D cell should deliver 5 to 6 A through a short circuit.
• The potential difference between the terminals should be 1.5 V when the cell is new. The value decreases as the cell ages. When the open-circuit potential difference falls below .75V, you should discard the cell.
The amount of current that a dry cell can deliver and still give satisfactory service depends on how long the current flows. For example, a No. 6 dry cell can supply a current of 0.125 A (its rated capacity) for several hours. A current greater than 0.125 A for a long period may damage the cell. But the same dry cell can supply a current of several amperes occasionally, for short periods of time, without damage.
Kinds of Primary Cells
One kind of primary cell is the air cell. It depends on oxygen from the air for depolarization. An example is the mercury oxide cell, also known as the Ruben cell or the RM cell.
The mercury cell delivers more electric energy than an ordinary dry cell of the same size.
Its most desirable characteristics are the following:
• high ratio of power output to weight,
• almost constant potential difference throughout its life
• long storage life at high temperature and humidity.
Another kind of primary cell is made and stored without electrolyte. The electrolyte is added to the cell just before it is put into service. This cell has a short active service life, but can be stored for long periods of time.
Secondary Cells
Storage batteries are made of secondary cells, often called storage cells. Secondary cells work in exactly the same way as primary cells—both have electrodes and an electrolyte. The main difference between primary and secondary cells is that secondary cells can be recharged.
The ability to be recharged comes from a difference in the electrodes. In the secondary cell, both the positive and negative electrodes change chemically during discharge. When the cell is fully charged, the two electrodes have different chemical compositions. As the cell discharges, both electrodes undergo chemical changes. The changes make the two electrodes alike. The more alike they become, the weaker the cell becomes.
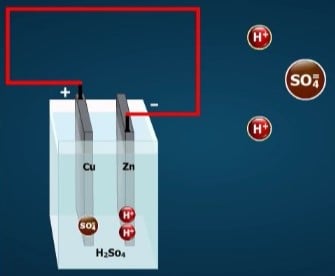
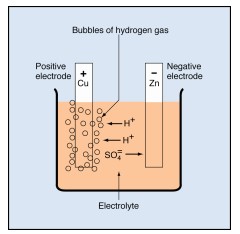
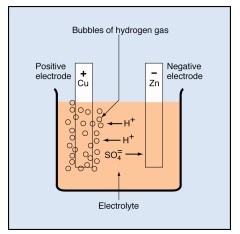
The lead-acid storage battery, used in automobiles and other vehicles, is made up of secondary cells. When the cells are fully charged, the negative electrode is made of lead (chemical symbol, Pb). The positive electrode is made of lead dioxide (PbO₂). The electrolyte is a strong solution of sulfuric acid (H₂SO₄), which consists of hydrogen ions (H+) and sulfate ions (SO₄).
As the cell delivers electricity, the negative electrode changes from lead to lead sulfate(PbSO₄). The positive plate changes from lead dioxide to lead sulfate. The sulfate (SO₄) comes from the electrolyte, leaving the electrolyte weaker. As the two electrodes become more and more alike and the electrolyte becomes weaker, the cell loses its ability to produce electricity.
During recharging, the chemical reactions inside the cell are reversed. The sulfate leaves the electrodes and returns to the electrolyte. The negative electrode changes back to lead, and the positive electrode changes back to lead oxide. Thus, the cell returns to its original condition and is able to produce electricity again. Recharging storage cells is an important part of battery maintenance.
A storage battery can deliver a large current immediately—almost any amount required. It can be carried wherever it is needed, although it is more difficult to carry than a battery made of primary cells.
There are two basic kinds of storage cells in use today:
• the lead–acid cell
• the nickel–cadmium cell.
Each of these cells has specific advantages over the other, and therefore is better for specific applications.
Storage batteries are classified according to their size. The automobile battery is a good example of the portable type that one person can carry. Batteries of this size are used for starting engines and for operating portable emergency lighting systems.
A large group of heavy-duty storage cells connected together make up a motive-power storage battery. Such a battery is big and heavy. It is commonly used for running forklift trucks, personnel carriers, and mine cars, and for providing power for various industrial applications.
Motive-power storage batteries have larger cells and use heavier electrodes than automotive batteries. They are assembled in hard-rubber jars set in steel trays. Trays of different sizes can hold a 12 V, 200 ampere-hour battery, or a battery with a 1000 ampere-hour capacity weighing several tons. Because of their heavier construction, motive-power batteries last from 3 to 6 years. Lighter storage batteries would soon fail.
Stationary Batteries
Some storage batteries cannot be moved easily from place to place. Because they stay in one place, they are called stationary batteries. They are used for operating switching equipment in electric-power stations when the normal source of power is interrupted. Stationary batteries are also used in hospitals for emergency lighting in case of a power failure.
Some stationary batteries weigh hundreds of pounds. They are able to operate for long periods of time. A typical battery may have 100 cells or more. The cells are arranged on racks so they can be checked individually.
A plant fire-alarm system is an example of the use of stationary storage batteries. The system normally operates on the regular power system in the plant. But a storage battery provides power to operate the alarm system if the regular power fails during a fire. The battery takes over immediately if the power fails.
A typical battery for this use is rated at 48V and 100 ampere-hours. It can provide at least 72 hours of operation. The battery also provides power for emergency lighting and the master control center.
Stationary batteries are built much like motive-power batteries. That is, they are heavier and have more rugged components than smaller storage batteries. One distinct feature of a stationary battery is the cell containers. They are made of glass or clear plastic to permit visual checking of the level.
Stationary batteries have a long life because they operate under almost ideal conditions. For example, the temperature is normally under 30°C or 80°F. When stationary batteries are used for emergency service, they must be kept fully charged and ready for immediate use. For this reason, each battery installation should include a battery charging unit.
Either lead-acid or nickel-cadmium cells may be used in stationary batteries. The choice of which kind to use depends on several factors. The most important factors are the service required and the conditions under which the battery will be used.
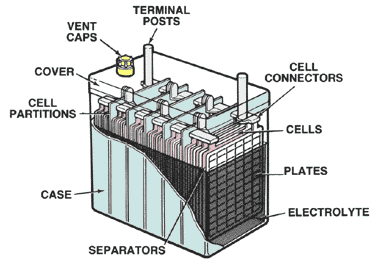
Construction of Storage Cells
All storage cells have the same basic construction. One kind may differ from another in minor details, but all have the same basic characteristics. The outer unit of the cell is a corrosion-resistant container, also called a jar. Jars are usually made of hard rubber, plastic, or glass.
Each cell has a set of positive and negative electrodes, called plates. The plates are arranged so that there is one more negative plate than positive plates. The assembly looks like a multilayer sandwich with a negative plate at each end of the stack.
The plates are insulated from one another by thin sheets called separators. The separators prevent short circuits between the negative and positive plates. A separator may be made of wood, rubber, glass, or plastic. It is to permit the electrolyte to flow through.
The complete assembly of positive and negative plates and separators is called an element. The positive plates are held together by a conducting bar or rod that ends at the positive terminal outside the cell. The negative plates are also connected by a conducting bar or rod that ends at the negative terminal. Terminals are usually identified by positive (+) and negative (–) signs. Some terminals are also color-coded, with red for the positive and green or blue for the negative.
A sealed cover over the element and container keeps out dirt and other foreign matter that could affect the cell’s operation. The cover also reduces evaporation of the electrolyte. A removable plug, called a vent plug, in the cover allows for testing and adding electrolyte. The vent plug may also have a small hole to permit the escape of hydrogen gas. Gaskets and seals prevent the escape of electrolyte and the entry of impurities.
A battery is an assembly of cells connected in series or parallel. The number of cells depends on the electrical needs of the application. A single lead-acid cell produces a potential difference of about 2V between the terminals. Therefore, a 6V battery has three cells, a 12V battery has six cells, a 120 V battery has 60 cells, and so on.
Other kinds of secondary cells produce different potential differences. As a result, different kinds of batteries have different numbers of cells, even though they produce the same potential difference between the terminals.
Proper maintenance of storage batteries includes keeping the terminals and connections clean. You must also keep the electrolyte at the proper level and strength, and keep the cell covers clean.
Rating Storage Batteries
The electrical characteristics of a battery determine whether it is suitable for a specific application. Storage batteries are rated in terms of their capacity, expressed in ampere-hours, or by the total number of plates in all the cells.
The most common method of rating storage batteries is by their capacity in ampere-hours. A rating of 1 ampere-hour means the battery can supply a current of 1 A for 1 hour. A battery is rated according to the number of ampere-hours it will furnish during a given period of time. For example, an 80 ampere-hour battery may be rated to supply 10A continuously for 8 hours if fully charged.
Although an 8 hour discharge period is common, some batteries are rated for other time periods. For example, the period may be 5, 4, or even 1 hour. The 8 hour period is common, partly because the standard working shift is 8 hours long in most plants.
A 400 ampere-hour battery may be rated to deliver 50A continuously for 8 hours. If the current is greater than 50A, the ampere-hour capacity of the battery is 400. For example, if the 400 ampere-hour battery is required to discharge its capacity in only 1 hour, its rating may drop by 50%. That is, the battery can be expected to deliver only 200A during the hour. The battery would probably be ruined in doing so.
You can easily calculate the rating required in a battery for a specific application. You just multiply the current required in the load by the length of time the current must flow before you recharge the battery. In mathematical terms the equation is written in the following way:
R = I ✕ t
where R = rating, in ampere-hours
I = current required, measured in amperes
t = time, measured in hours
For example, suppose a load requires current of 10A for 10 hours. Using the equation, you can calculate that you need a battery with a rating of at least 100 ampere-hours:
R = I ✕ t
= 10 A ✕ 10 hours
= (10 ✕ 10) ampere-hours
= 100 ampere-hours
The watt-hour capacity of a battery is a measure of its total energy content. To calculate the value, you multiply the ampere-hour rating by the average potential difference between the terminals during discharge. For example, if a battery delivers 50 ampere-hours and maintains an average potential difference of 6V, the total energy delivered is 300 watt-hours.
The potential difference between the terminals of a battery depends on several factors, including the current delivered, the concentration of the electrolyte, the temperature, and the number and condition of the cells. The physical size of a cell does not determine the potential difference between its electrodes. Only the materials in the cell affect its potential difference. For example, all lead-acid storage cells produce about 2V.
As a cell discharges, the potential difference between its terminals decreases. The decrease is caused by an increase in the internal resistance of the cell itself. The loss of potential difference is called the voltage drop. It increases as the current increases.
As the discharge continues, the potential difference becomes lower. The value may eventually fall so low that the battery cannot serve its intended purpose. If current continues to flow, the battery finally reaches a minimum value called the final voltage. Final voltage varies with the rate of discharge, and is lower at higher current. When the potential difference of the individual cells falls to this value, it is time to recharge the battery.
The final voltage of a lead-acid battery is about 1.85V per cell if the rate of discharge is low. If the discharge rate is high, the final voltage may be as low as 1.0V per cell. As a rule, 1.85V per cell is a reasonable final voltage for most storage batteries.
During recharging, lead-acid storage batteries produce hydrogen gas. This gas is extremely flammable. An open flame, a slight spark, or even a smoker’s glowing tobacco can ignite the gas and cause an explosion. The explosion can break the battery case and throw acid on anyone nearby.
If you get acid on your skin or in your eyes, you must take action immediately. Wash the acid off your skin or out of your eyes with a flood of clean water. Use an eye-wash fountain if one is available nearby. Then get medical attention as soon as possible.
The amount of current a cell can deliver is limited by the internal resistance of the cell. Internal resistance is partly a result of local action and partly a result of polarization.
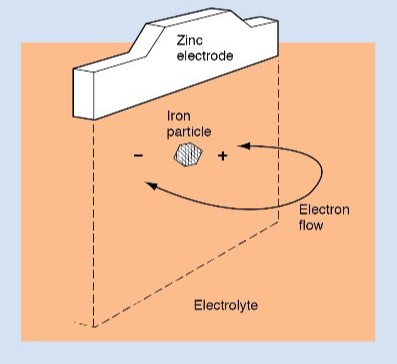
Anything that can be done to reduce internal resistance is desirable. In a storage cell, internal resistance can be reduced by putting the plates very close together. The plates are made as large as possible to increase the surface area exposed to the electrolyte. Vent plugs that permit the escape of gas formed during the cell’s action also reduce internal resistance.
The life of a storage battery is measured not only in months and years, but by the number of times it can be discharged and recharged. The combination of one discharge and one recharge is called one cycle. The average cycle life of individual batteries is between 1300 and 1500 cycles. Some storage batteries must be replaced after only 2 years of service, if they are charged and discharged often. Other batteries last longer.
Summary
Batteries have many uses in the plant. A battery is made up of two or more cells connected together. Each cell has two electrodes and an electrolyte. There are two basic kinds of electric cells—the primary cell and the secondary cell. The primary cell cannot be recharged. The secondary cell can be recharged and used again. Both kinds of cells are either wet or dry, depending on whether the electrolyte is a liquid or a paste.
The dry cell is the most widely used primary cell because it is portable, has no spillage problems, is inexpensive, and is reliable when used under the proper conditions.However, dry cells do not have a long storage life and are suited only to intermittent use. The potential difference of a dry cell is about 1.5 V. Dry cells should be stored in a dry place at a temperature of about 70°F.
Secondary cells are connected together to make up storage batteries. The two basic types of storage cells are the lead-acid cell and the nickel-cadmium cell. Storage batteries are classified according to their size, and rated in terms of their capacity. Motive-power storage batteries and stationary batteries are used in applications where superior life and heavy-duty output are required.