Electrochemical Action
Batteries are a completely independent and self-contained source of electrical energy. They can be moved from place to place as needed. When properly maintained they are dependable and reliable. For these reasons they have many uses in industry.
Understanding the electrochemical action and how battery cells work will help with proper maintenance. This article explains cell and battery characteristics, electrochemical action, and cell chemistry. As well as the process of electrolysis, and how it is used in various industrial applications.
History Of Batteries
Until batteries were discovered by an Italian scientist named Alessandro Volta, the only man made electricity was static electricity that was generated in special machines. Alessandro Volta performed experiments that resulted in today’s modern batteries.
Volta discovered that he could generate a steady flow of electricity, in large quantities, by chemical means. He placed two strips of different metals in a glass cup filled with acid or a solution of salt or lye. Then he connected the upper ends of the strips with a wire. Electricity flowed through the wire, from one strip to the other, as long as the chemical reaction continued.
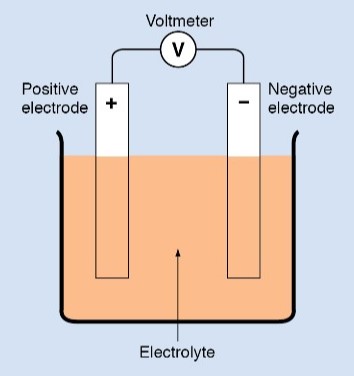
The liquid in the cup is called an electrolyte. The basic arrangement is called an electrochemical cell, or a voltaic cell in honor of Volta. The unit for measuring potential difference is called the volt. Like the voltaic cell, it is also named in honor of Volta.
A battery is made by connecting two or more voltaic cells together. They may be connected either in series or in parallel.
Modern cells and batteries have been developed to serve many kinds of needs. They look very different from Volta’s experimental cells, but they work in the same basic way. Each cell consists of two different kinds of metal in a liquid or semiliquid electrolyte.
Battery Characteristics
All batteries and all cells work by electrochemical action. During this process, chemical energy decreases as the cell produces electrical energy. When the chemical energy of the cell has been exhausted, the cell can no longer produce electricity.
Some voltaic cells are made to be thrown away after their chemical energy has been exhausted. Examples include the cells used in flashlight and calculator batteries. These disposable cells are called primary cells. Cells that are rechargeable, like the storage batteries in automobiles, are called secondary cells.
The total amount of energy a cell can produce depends on how much metal and how much metal it contains.
The total amount of energy a battery can produce depends on how many cells it has.
The life of a cell or battery is generally measured in ampere-hours. This value is the amount of current the cell or battery can deliver for a measured amount of time. The value indicates the total amount of charge the cell or battery can deliver as shown below:
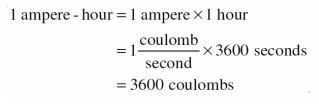
If you multiply the number of ampere-hours by the potential difference between the terminals, the result is the total amount of energy the cell or battery can produce:
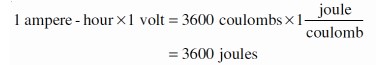
The best battery for a specific use depends on the kind of electrical service required and the conditions of use. For example, the kind of electrical service includes the potential difference required, the current required, and the length of operating time. The conditions of use include whether the battery is to be stationary or movable, the range of operating temperatures, and the atmosphere surrounding the battery.
Electrochemical Action
To understand how a battery works, you must understand electrochemical action—a combination of electrical and chemical processes.
An electrical cell consists of two elements—a pair of electrodes and an electrolyte.
• Each electrode is a strip, a bar, or a plate of conducting material, usually made of metal or carbon. The electrodes must be chemically different from each other.
• An electrolyte is a liquid or a paste that can conduct electricity. It must react chemically with one of the electrodes.
Two electrodes are placed in the electrolyte. The electrodes do not touch each other. The electrolyte reacts with each electrode individually. Because of these reactions, a potential difference is established between the electrodes.
The chemical reaction at one electrode releases electrons from the material, giving that electrode a positive charge. The released electrons enter the electrolyte, and drift toward the other electrode. When the electrons reach the other electrode, they give it a negative charge. The cell now has one positive electrode and one negative electrode. This difference in charge creates a potential difference between the electrodes.
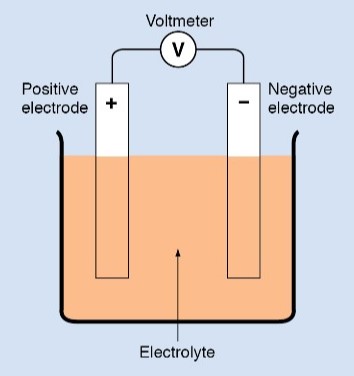
When the electrolyte has all the extra electrons it can hold, the chemical reaction stops. No further chemical reaction occurs until a complete circuit is made by connecting the positive and negative electrodes externally, creating a current path.
When the current path is complete, an electric current flows between the two electrodes.
Chemical action continues until the material of the negative electrode is completely used up or the electrolyte is no longer chemically effective. When all electrochemical action stops, the cell is said to be discharged.
Cell Chemistry
In order to understand electrochemical action, you must understand a little basic chemistry. You may remember most or all of the chemistry you need, but if not the following paragraphs will summarize what you need to know.
All matter consists of atoms. In some materials, all the atoms are alike. Such a material is called an element. Copper is an example of an element. It is impossible to break copper, or any other element, into two or more different materials by chemical means. The smallest unit in a piece of pure copper is a copper atom.
Some materials are made of two or more different kinds of atoms chemically joined together. The atoms join together in units called molecules. All the molecules in a pure substance are identical. They all consist of the same numbers of the same kinds of atoms.
The largest molecules consist of thousands of atoms. Yet they are too small to see, even with the aid of the most powerful optical microscope. Many molecules consist of only two or three atoms joined together. A material made of two or more kinds of atoms joined together chemically is called a compound.
All matter consists of various combinations of the atoms of 92 elements. A total of 103 elements are known, but 11 of them are unstable. They exist for only fractions of a second during nuclear reactions, and then divide into two or more simpler atoms.
Every element has an atomic number. This number equals the number of protons in the nucleus of the atom. The number of protons influences the number and arrangement of electrons surrounding the nucleus, and the arrangement in turn determines the chemical properties of the element.
Chemical symbols have been created for all the elements. The symbol consists of the first letter of the element’s name, and possibly one additional letter. The first letter is always a capital. Chemists use these symbols in writing chemical formulas and for identifying elements in diagrams or elsewhere. The letters in a chemical symbol may come from the modern English name of the element, from an older English name, or from a Latin name.
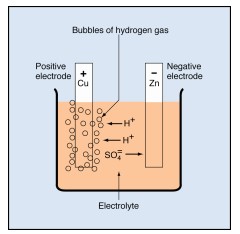
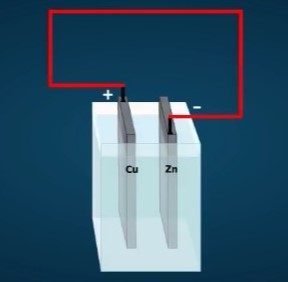
You can now use these ideas about chemistry to understand the voltaic cell. Suppose the cell consists of copper and zinc electrodes in an electrolyte of sulfuric acid. The parts are arranged as shown below. The electrodes are identified by their chemical symbols. The copper electrode is labeled Cu in the diagram, and the zinc electrode is labeled Zn.
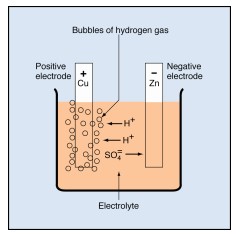
The chemical formula for the electrolyte is H₂SO₄. This formula tells you that each unit of the acid consists of two hydrogen atoms (H), a sulfur atom (S), and four oxygen atoms (O).
The acid does not exist as a collection of molecules. Like all acids, it ionizes.
That is, its molecules divide into parts, with each part carrying an electrical charge.
Sulfuric acid molecules divide into two hydrogen ions (labeled H+) and one sulfate ion( SO₄).
• Each hydrogen ion lacks the electron required to make it a hydrogen atom. That is, it consists of a single proton, carrying one positive charge.
• The sulfate ion carries two extra electrons, one from each of two hydrogen atoms. Thus, it carries a double negative charge.
The ions are free to move in the electrolyte. As they move, they carry positive and negative charges between the electrodes.
In the voltaic cell, the sulfate ions combine chemically with atoms of zinc, forming molecules of zinc sulfate (ZnSO₄). In doing so, the sulfate ions give up their extra electrons to the zinc electrode, giving it a negative charge.
The hydrogen ions drift toward the copper electrode. As they arrive, each one removes an electron from the copper. Each electron removed changes a hydrogen ion to a hydrogen atom. It also leaves the copper electrode with a positive charge.
The newly formed hydrogen atoms combine in pairs to form molecules of hydrogen (H₂). The molecules collect and form bubbles of hydrogen gas that rise to the surface of the electrolyte and escape.
Tiny bubbles of hydrogen gas collect on the copper electrode. The bubbles have an insulating effect, which slows down the chemical reaction and reduces the cell’s output capacity. This undesirable condition is called polarization. Other chemicals can be added to the electrolyte in a cell to decrease polarization. Decreasing the polarization is called depolarization.
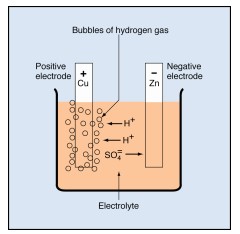
While the current is flowing, the zinc changes into zinc sulfate and hydrogen gas continues to form. This process stops when the zinc electrode is consumed or when bubbles of hydrogen completely cover the copper electrode. Both conditions prevent further electrochemical action.
Electrolysis
Electrochemical action has other important industrial applications. In a voltaic cell, the chemical reaction destroys the negative electrode. But in a reverse process called electrolysis, the negative electrode builds up. Material is added to the electrode as electricity is forced backward through the cell.
Electrons flowing into the negative electrode force metal ions in the electrolyte to change to atoms. The metal ions in the electrolyte have a positive charge, because they lack electrons. When the ions receive the electrons they lack, they change to metal atoms and collect on the surface of the negative electrode.
Electrochemical action in a voltaic cell produces direct current. Electrolysis uses direct current. The current may come from another voltaic cell or battery, or it may come from a generator or a rectifier.
There are many industrial uses of electrolysis. Examples include the purifying of metals, the plating of one metal on another during manufacturing, and the separating of desirable metals from raw materials.
Electroplating
Electroplating is the process of covering one metal with a thin layer of another metal. Usually the purpose is to protect or decorate the underlying material.
Chrome plating is a good example of this process. The layer of chromium prevents the steel underneath from rusting and gives the part an attractive appearance. Another example is copper plating the carbon brushes used in dc motors and generators.
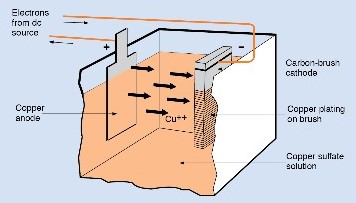
In the process of plating an iron object with copper, Two electrodes are required. The iron object serves as the negative electrode, and a piece of copper serves as the positive electrode. The positive electrode is called the anode. The negative electrode is called the cathode. The electrolyte is a solution that contains copper ions.
When electrons flow from the cathode to the anode, they change the copper ions in the electrolyte into copper atoms. These atoms form a layer of copper on the iron cathode. At the anode, the opposite process occurs. Atoms of copper lose their outer electrons and become copper ions in the electrolyte. These ions replace those lost from the electrolyte at the cathode until the anode gradually disappears.
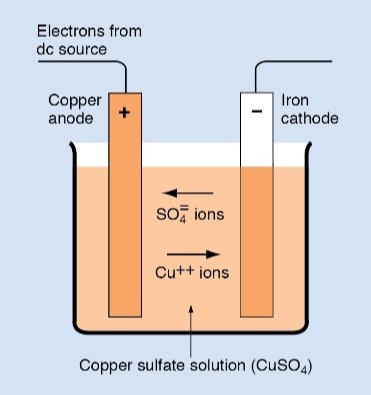
The total amount of copper remains unchanged during the plating process. The amount of copper gained at the cathode equals the amount lost at the anode. The electrolyte serves as a medium to transfer the copper from the anode to the cathode.
The anode must be made of the metal to be plated, and the electrolyte must contain ions of that metal. For example, plating nickel onto another metal requires an anode made of nickel. The electrolyte must consist of a material that contains nickel ions.
Extracting and Refining Metals
Metals found in nature are usually chemically combined with other elements. For example, aluminum is combined with oxygen in a compound called alumina (Al₂O₃). Electrolysis is used for extracting the aluminum from the oxygen. The process requires a great deal of electricity—about 6 kilowatt-hours per pound of aluminum extracted. But it is the least expensive method of extracting the aluminum.
The last step in purifying copper and other metals is often a process called electrolytic refining. In electrolytic refining, the impure metal forms the anode. When electric current is passed through the electrolyte, the desired metal atoms in the anode change to ions, enter the electrolyte, and move toward the cathode. When the ions reach the cathode, they change back to metal atoms and collect on the surface of the cathode. The impurities remain behind and fall to the bottom of the tank.
Electrolytic Corrosion
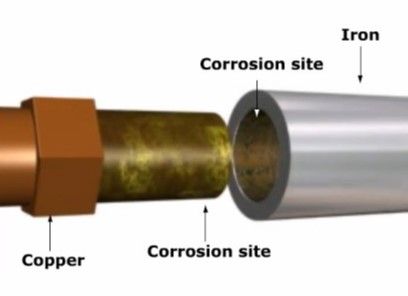
Electrolysis often causes rapid corrosion of metals. This effect is especially common in cables and piping. Corrosion also occurs in metal buildings, just above or below ground level.
In both cases, the corrosion results from the formation of small voltaic cells. The cells consist of different materials in electrical contact through an electrolyte formed naturally of water and dissolved minerals in the soil.
Rapid corrosion occurs in piping and cables when two different metals make contact in the presence of an electrolyte. For example, joining copper piping to iron piping creates a voltaic cell. The copper becomes the cathode, and the iron becomes the anode. The anode is rapidly destroyed by the electrochemical reaction. The joint may fail in just a few years.
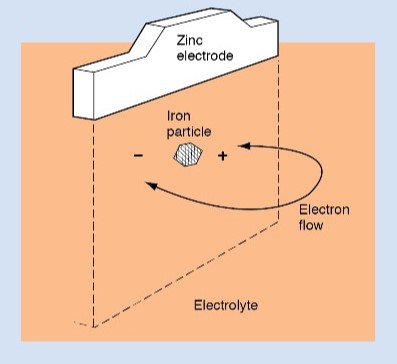
If two different metals are buried in the ground and make electrical contact, electrolytic action may take place. The metal that serves as the anode is destroyed rapidly.
The extent of electrolytic corrosion, and how rapidly the damage occurs, depends on several factors. The kind of soil, the kind of metals and their sizes, and the amount of moisture in the soil all influence electrolytic action.
Electrolytes
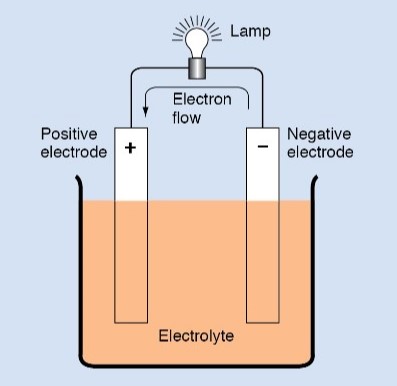
An electrolyte is a solution that can conduct an electric current. In addition, it must react chemically with the material on one of the electrodes used. The electrolyte used in most voltaic cells is a solution of sulfuric acid. However, some cells have an alkaline electrolyte, usually potassium hydroxide.
Regardless of the kind of electrolyte in a cell, the electrochemical action is always the same. That is, electrons flow through the electrolyte from the anode to the cathode. When a complete circuit is formed by connecting the two electrodes outside the electrolyte, electrons flow from the cathode to the anode.
Clearly, the electrolyte is an important factor in battery performance. The performance of the electrolyte is affected by temperature, impurities, chemical changes, and its concentration. The effects of these conditions are explained in the following paragraphs.
Temperature. The ideal operating temperature range of most industrial batteries is 15 to 25°C (60 to 80°F). To achieve the best performance and life, most batteries should not be used in temperatures above 38°C (100°F). The effect of temperature is more noticeable near the freezing point of water. The effect is sometimes described as “numbing” the battery, reducing its operation. Damage is not usually permanent, however.
Impurities and chemical changes. Most electrolytes are quite clean. They contain few impurities. If too many impurities are present, especially if they are certain minerals or metals, they set up voltaic cells within the electrolyte. This condition is called local action. The effect is like a short circuit within the cell.
Even when a battery is not being used, local action continues to use up the negative electrode. When you add water to the cells of a storage battery, you should use only distilled or purified water in order to reduce local action.
Concentration. The concentration of a liquid electrolyte can be measured in terms of its specific gravity. The specific gravity of a material is its density compared to the density of water:
According to this definition, pure water has a specific gravity of 1.000. Pure sulfuric acid has a specific gravity of 1.800, meaning the acid is almost twice as dense as pure water.

Sulfuric acid is diluted with water to make the electrolyte for automotive storage batteries. The usual mixture is four parts of acid to two parts of water. The mixture has a specific gravity of 1.300. In practice, a value of 1.275 is considered full strength.
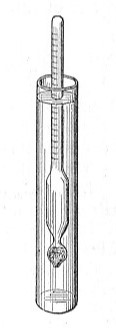
As each cell in the battery produces electricity, the chemical reaction produces water. The water dilutes the sulfuric acid and decreases its specific gravity. Therefore, you can test the condition of the cell by measuring the specific gravity of the electrolyte. If the specific gravity decreases to 1.100, the cell is discharged.
Choosing a Battery
You need to consider many factors in order to choose the proper battery for an application. These factors include charge and discharge characteristics at various temperatures, the size, the weight, the durability, the cost, and the maintenance requirements. You can get detailed information from the manufacturers of the batteries before making a final selection.
Battery Power
Very few manufacturing plants or other facilities can operate without using a battery for some purpose.
• Automobiles, trucks, diesel locomotives, aircraft, and other machines with
engines require batteries for their operation.
• Hospitals rely on batteries to provide emergency service in corridors, operating rooms, and other critical areas if the regular power fails. Hospitals also have battery-started generators to supply electricity automatically in case of a power failure.
• In an industrial plant, battery-powered lighting and alarm systems help protect workers if the power fails during an emergency.
The reliability of all batteries depends on proper maintenance.
Summary
Batteries have many uses in the plant. A battery is made by connecting two or more cells together. Each cell has two different kinds of metal, called electrodes, in a liquid or semiliquid electrolyte. The total amount of energy a cell can produce depends on how much metal and how much electrolyte it contains. The total amount of energy a battery can produce depends on how many cells it has.
Some cells cannot be recharged. These are called primary cells. Cells that are rechargeable are called secondary cells. Examples of primary cells include those used in flashlights and in calculators. A storage battery in an automobile is an example of a secondary cell.
Besides its importance in cells, electrochemical action has other industrial uses. An electrochemical process called electrolysis is used in industry in purifying metals, in plating, and in separating metals from raw materials.
In order to choose the proper battery for an application, you must consider several factors. These include charge and discharge characteristics at various temperatures, the size, the weight, the durability, the cost, and the maintenance requirements. Always select a battery that meets your needs.

